Since their introduction more than 40 years ago, interfacially polymerized reverse-osmosis membranes have become the leading technology used in desalination worldwide. However, despite the popularity of these membranes, scientists long have had an incomplete understanding of precisely how water passes through them.
In "Nanoscale control of internal inhomogeneity enhances water transport in desalination membranes," a recently published article in the journal Science, a team of academic and industry researchers document how minute differences in density within RO membranes affect their performance. The findings are expected to boost membrane performance significantly, potentially reducing the amount of energy required to operate membranes and thereby cutting the cost of a critical treatment process at the center of desalination and, increasingly, water reuse efforts.
Used in high-pressure treatment applications, an interfacially polymerized membrane includes an extremely thin film at the interface between a water-soluble diamine solution and an acid chloride solution. The resulting combination of materials produces a membrane that is relatively permeable to water but not to most impurities.
Exactly how water flows through membranes has not been well understood. “Reverse osmosis membranes are widely used for cleaning water, but there’s still a lot we don’t know about them,” said Manish Kumar, Ph.D., P.E., an associate professor in the Civil, Architectural, and Environmental Engineering Department at the University of Texas at Austin, in a Dec. 31 news release issued by the university. Kumar co-led the research team that recently published its findings regarding membrane performance. “We couldn’t really say how water moves through them, so all the improvements over the past 40 years have essentially been done in the dark,” Kumar said.
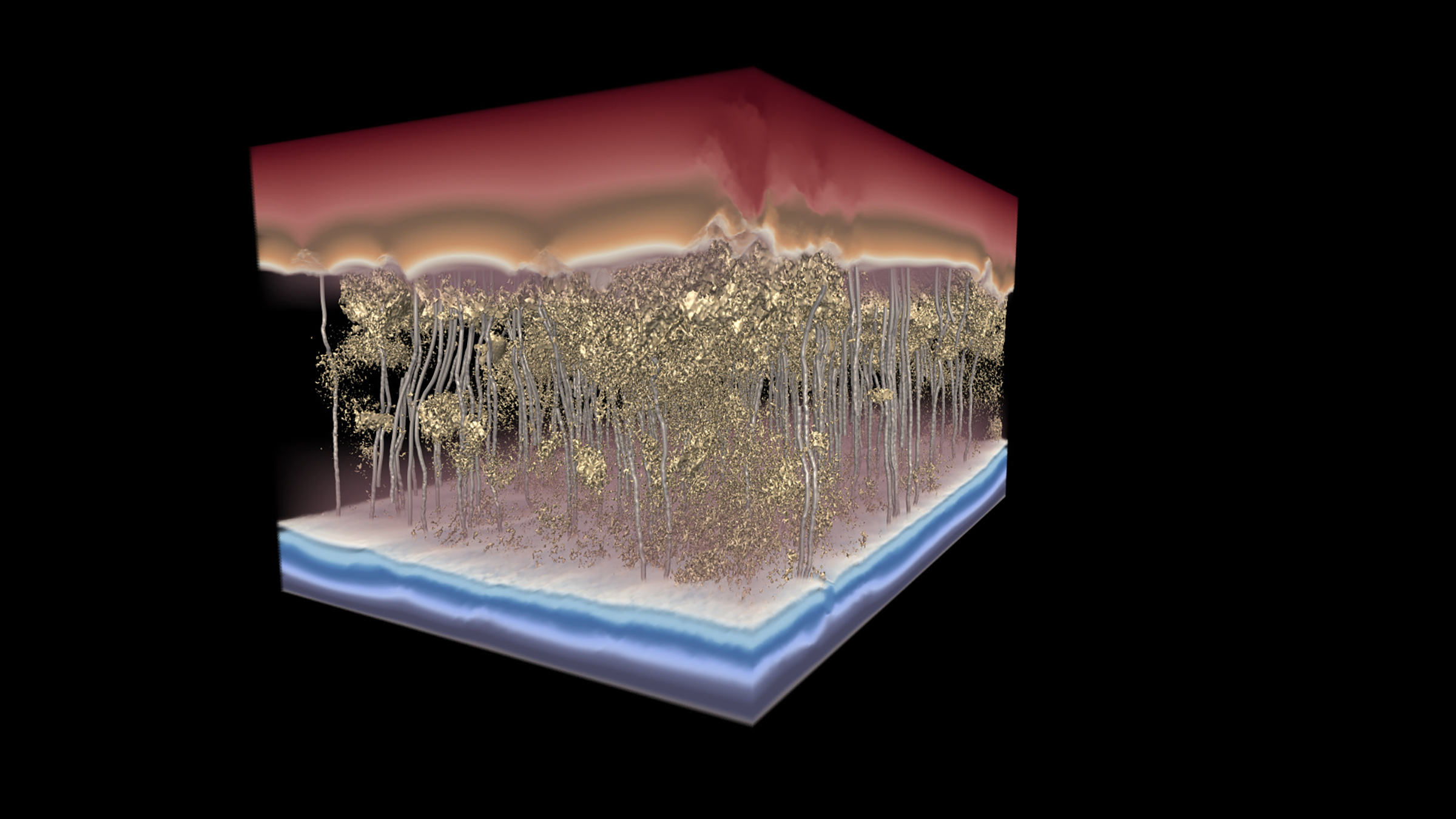
For example, membrane thickness was assumed to be a key factor in permeability, with thicker membranes thought to be less permeable. However, scientists at DuPont Water Solutions, a manufacturer of RO membranes, noticed something odd: Thicker membranes sometimes had greater permeability than thinner membranes. This finding prompted the DuPont scientists to partner with the academic researchers to try to solve the apparent conundrum.
In addition to DuPont and the University of Texas at Austin, the researchers hailed from Pennsylvania State University, Iowa State University, and the Dow Chemical Co. Funding for the research came from the National Science Foundation and DuPont.
Using multimodal electron microscopy, the researchers created 3D reconstructions of the internal structure of RO membranes at the nanoscale level. In this way, the research team was able to note variations in density within the membranes at a spatial resolution of approximately 1 nm. The researchers then modeled how water passes through the membranes.
Ultimately, the researchers determined that density is more important than thickness when it comes to membrane performance.
“We found that how you control the density distribution of the membrane itself at the nanoscale is really important for water-production performance,” said Enrique Gomez, Ph.D., a professor in the Department of Chemical Engineering at Penn State, in a Dec. 31 news release issued by the university. Along with Kumar, Gomez co-led the research team, which published its findings in the Science article.
By using the analytical techniques described in the Science article, membrane manufacturers will better understand how their production process affects membrane performance, Kumar told Civil Engineering.
Currently, manufacturers test their membranes to ensure proper performance, but the link between manufacturing process and membrane behavior is not always clear. “You don’t know what the chemistry did to lead to the performance you get,” Kumar says. “What we provide in this paper is a way to connect the two.”
The analytical approach developed by the research team will enable membrane makers to boost the performance of their products, Kumar says. “You can see what happened to the structure of the polymer, and then you know why your membrane is behaving a certain way,” he says. “It helps you design for the most efficient membranes that you can make using this method.”
In the study, the research team demonstrated that membranes can perform much more efficiently when optimized in terms of their density. “In the actual membranes we tested, we had 30 percent improved productivity,” Kumar says. Such an increase in productivity would be expected to result in significant energy savings during water treatment, he notes. “It won’t be exactly 30 percent,” Kumar says. “It will be less because there are other factors involved. But that’s the scale we’re talking about in terms of energy reduction, tens of percentages.”




